Written by: Equities Research
~ Clinical candidates for reversal agent for antiplatelet drug and for pulmonary arterial hypertension ~Heart disease remains the leading cause of death for both men and women. About 610,000 people die of heart disease in the US every year according to the CDC, or about one out of every four deaths. About 735,000 people in the US have a heart attack each year, according to a report from the American Heart Association. Of these, 525,000 people experience a heart attack for the first time, while 210,000 people have a repeat attack.Patients who suffer heart attacks, or other acute coronary syndromes (ACS) in which blood flow to the heart is suddenly restricted, are frequently treated with antiplatelet drugs to prevent blood clots. Current guidelines issued by The American College of Cardiology, American Heart Association and European Society of Cardiology recognize a drug called ticagrelor as the preferred antiplatelet therapy for ACS. Ticagrelor is often given in combination with aspirin as dual therapy. A serious side effect for patients on ticagrelor, however, is an elevated risk of spontaneous bleeding. Further, patients on ticagrelor who need urgent surgery can't afford to wait the recommended five days for ticagrelor’s effect to dissipate. These patients face an increased risk of major bleeding during and after surgery.Malvern, Pennsylvania, based PhaseBio Pharmaceuticals (proposed Nasdaq: PHAS) is developing a novel reversal agent for ticagrelor called PB2452, for the treatment of patients on the antiplatelet drug who are experiencing a major bleeding event or those who require urgent surgery. The company recently completed a Phase 1 dose ranging clinical trial of PB2452 in healthy subjects, achieving rapid and complete reversal of ticagrelor’s antiplatelet activity within five minutes following initiation of infusion, and sustained reversal for over 20 hours in later dosing cohorts in which PB2452 was administered over an extended infusion period.PhaseBio's second candidate, PB1046, is a once-weekly treatment currently in a Phase 2b clinical trial for the treatment of pulmonary arterial hypertension, a progressive, life-threatening, orphan disease caused by vasoconstriction and structural deterioration of the pulmonary arteries, which can lead to heart failure and death. PB1046 is based on PhaseBio's proprietary elastin-like polypeptide, or ELP, technology, which also serves as the engine for the company's preclinical pipeline and long-term driver of potential growth.PhaseBio is offering 5,000,000 shares at $12 to $14 via Citigroup, Cowen and Stifel. At the midpoint of the filing range, it would be a $65 million initial public offering with a post-money market capitalization of $255 million. The largest shareholders include New Enterprise Associates (24.6% ownership post-IPO), Zeneca, a subsidiary of AstraZeneca ( AZN[NYE] - $37.47 ) (12.2%), Hatteras Venture Partners (11.0%), Johnson & Johnson Innovation - JJDC, a subsidiary of Johnson & Johnson ( JNJ[NYE] - $133.95 ) (7.5%) and Fletcher Spaght Ventures (4.7%).https://player.vimeo.com/video/139967572 PB2452 - reversal agent for ticagrelorRelated: Micro-Bubble WinnersPhaseBio intends to initiate a Phase 2a trial of PB2452 in healthy older subjects in the first half of 2019 in order to evaluate safety and efficacy of the potentially therapeutic doses and dosing regimens from the Phase 1 trial. Older adults exhibit more variability in drug response to ticagrelor and higher levels of baseline platelet reactivity compared to younger subjects, and they resemble the patient population most likely to be treated with ticagrelor and, therefore, potentially benefit from PB2452. Looking ahead, PhaseBio intends to pursue an accelerated approval pathway with the FDA.The company licensed PB2452 from MedImmune, a subsidiary of AstraZeneca, in November 2017. If PB2452 is successful, it will be a win-win for both companies. Ticagrelor, marketed by AstraZeneca, had worldwide sales of over $1 billion in 2017 and $609 million in the first half of 2018. PhaseBio is unaware of any other reversal agents approved or in clinical development for ticagrelor or any of the other antiplatelet drugs. The availability of a reversal agent could expand ticagrelor’s use by mitigating concerns regarding bleeding risk and uniquely position ticagrelor as the only oral antiplatelet drug with a reversal agent.ELP technology
PhaseBio's ELP technology extends the circulating half-life of proteins and peptides in the body while also providing a sustained-release mechanism, resulting in exposure of active molecules for periods of a week or longer from a single subcutaneous injection. The company believes that its ELP technology enhances solubility, stability and bioavailability, provides extended drug exposure and creates product candidates that are straightforward to manufacture and administer. PhaseBio's strategy is to apply ELP technology to proteins and peptides with well-characterized therapeutic activities but suboptimal half-lives in order to improve their pharmacokinetics, enable their use as pharmaceutical products and enable more convenient dosing regimens. To date, the company hasn't observed any serious adverse events in any of the over 500 patients in clinical trials of its ELP product candidates.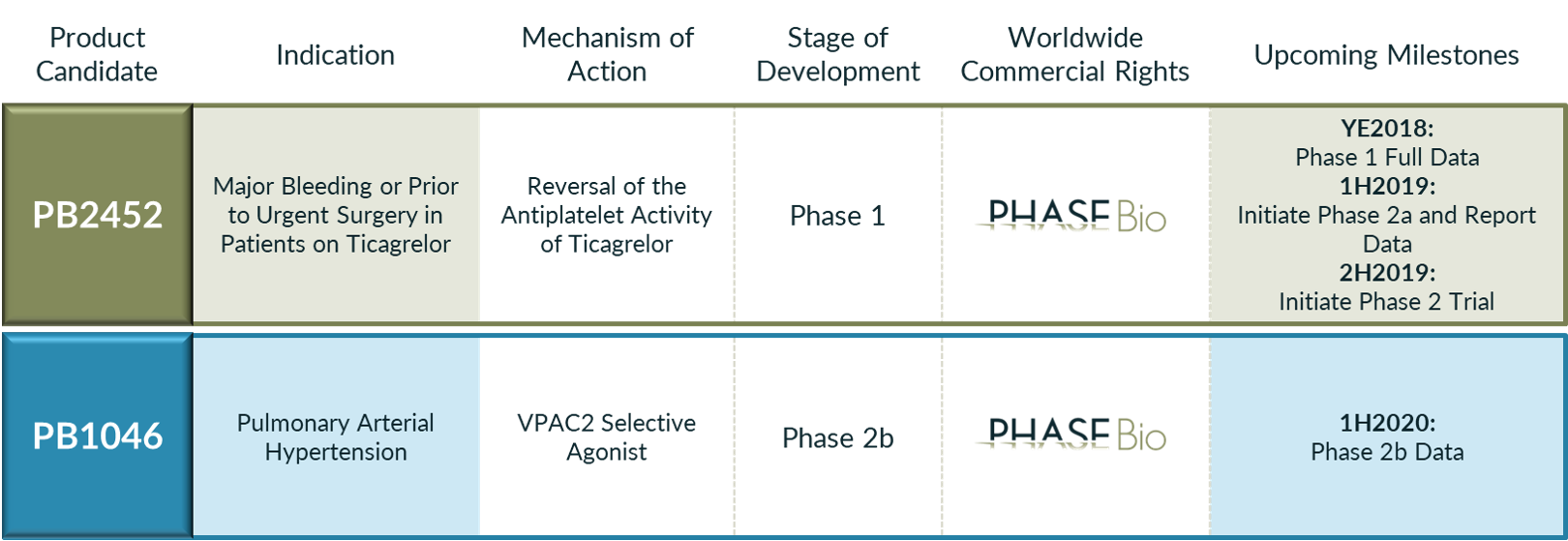 Source: PhaseBio Pharmaceuticals Management Jonathan Mow has been a biotech and pharma business development executive for 25 years. He has been CEO of PhaseBio for three years and was previously Chief Business Officer. Prior to PhaseBio, he was VP, Business Development, for Amylin Pharmaceuticals until its sale to Bristol-Myers Squibb ( BMY[NYE] - $57.61 ); co-founder and VP, Commercial and Business Development, of Corus Pharma until its acquisition by Gilead Sciences ( GILD[NGS] - $72.78 Trade ); and head of business development for PathoGenesis until its acquisition by Chiron, which was later acquired by Novartis ( NVS[NYE] - $83.15 ).Chief Medical Officer John Lee, MD, PhD, is a cardiologist with more than 15 years of combined medical practice and biopharmaceutical management experience. He was previously VP & Global Head of the Cardiovascular Center of Excellence at Quintiles Transnational ( IQV[NYE] - $124.26 ), where he led drug development and product lifecycle management. Prior to Quintiles, Dr. Lee held several positions at Bristol-Myers Squibb, including Executive Director, Head of Cardiovascular/Metabolic Therapeutic Area. DISCLOSURE: The author has no positions or any beneficial interest in, and has not received any compensation from, the companies mentioned in this article.
Source: PhaseBio Pharmaceuticals Management Jonathan Mow has been a biotech and pharma business development executive for 25 years. He has been CEO of PhaseBio for three years and was previously Chief Business Officer. Prior to PhaseBio, he was VP, Business Development, for Amylin Pharmaceuticals until its sale to Bristol-Myers Squibb ( BMY[NYE] - $57.61 ); co-founder and VP, Commercial and Business Development, of Corus Pharma until its acquisition by Gilead Sciences ( GILD[NGS] - $72.78 Trade ); and head of business development for PathoGenesis until its acquisition by Chiron, which was later acquired by Novartis ( NVS[NYE] - $83.15 ).Chief Medical Officer John Lee, MD, PhD, is a cardiologist with more than 15 years of combined medical practice and biopharmaceutical management experience. He was previously VP & Global Head of the Cardiovascular Center of Excellence at Quintiles Transnational ( IQV[NYE] - $124.26 ), where he led drug development and product lifecycle management. Prior to Quintiles, Dr. Lee held several positions at Bristol-Myers Squibb, including Executive Director, Head of Cardiovascular/Metabolic Therapeutic Area. DISCLOSURE: The author has no positions or any beneficial interest in, and has not received any compensation from, the companies mentioned in this article.


